Enterprise-Proven AI for
the Entire Content Workflow
Increase speed. Lower costs. Ensure compliance.
Increase speed. Lower costs.
Ensure compliance.
Every Second Counts
Healthcare moves fast, and so must we. With drug costs under pressure and patents running out, life sciences companies need to cut costs without sacrificing production quality or compliance. To stay competitive, we have to get better at quickly reaching doctors and patients with the right information.
Lithero AI Agents
We can proudly say that Lithero is the best-in-class AI for Life Sciences Marketing Teams. Pharma leaders call our agents 'magic.'
Lithero AI Agents
After 30+ combined years of Life Sciences industry experience, PhD. level research in machine learning, 8 years of iterative development, and customer-proven results, we can proudly say that Lithero’s agents are the best-in-class AI for Life Sciences Marketing Teams. Lithero is helping some of the world’s leading healthcare organizations compress timelines, drive personalization at scale, and unlock real growth.
Book a DemoClaims
Saves research time by auto-learning and maintaining a searchable library from approved brand materials
Annotate
Lessens the workload of manually tracing claims back to substantiated references
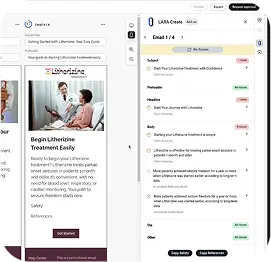
WriteBETA
Helps writers create strategic, personalized, and compliant copy in seconds
DesignBETA
Eases the burden on marketers to quickly demonstrate their concepts and execute without delay
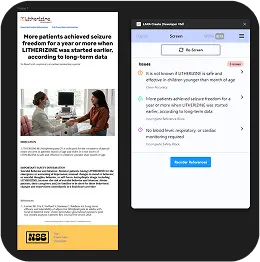
Screen
Empowers teams to focus on what matters—creating compelling materials instead of manual compliance checking
Integrations
Brings the power of Lithero’s Agents directly to other tools you already use.
End to End Efficiency
Lithero AI Agents are designed for use throughout the content delivery process, providing precious time savings at each step that add up.

Joan
Account Manager

Claire
Head of Brand

Adeline
Copywriter

Chris
Designer

Lilly
Editor

Claire
Head of Brand QC

Andy
Submission Rep
AI that’s secure, scalable, and built for your brand.
Lithero Agents learn and grow with you.
Lithero delivers enterprise-ready agentic AI that checks every box—from security to scalability—so your teams can get up and running fast, with zero headaches and measurable 14X ROI.
Lithero AI Agents
Rather than solving for isolated use cases, Lithero seamlessly integrates AI across your entire end-to-end content workflow—connecting content generation, claims management, initial design, reference annotation, and MLR review into one intelligent, streamlined system.
BOOK A DEMOClaims
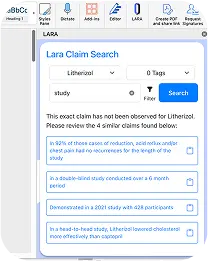
Find Approvable Claims with Confidence
With Claims, extracting approved claims is as easy as one click. Easily search through every claim that’s ever been used to find exactly what you need- without sifting through an outdated spreadsheet.
Fast Setup, Zero Manual Work
Get your claims library built and live in just 3 business days—no spreadsheets, no manual tagging, no tedious cataloging.
Automated Claim Extraction from Approved Materials
Claims are intelligently pulled from your already-approved content, ensuring accuracy and consistency across all your assets.
Expert Support from Day One
Our dedicated customer experience team provides hands-on onboarding, training, and ongoing support to ensure long-term success and adoption.
- 80% of time saved finding where claims have been used before
- 90% of time saved creating and maintaining claims matrix spreadsheets
- Single source of truth that is always aligned with your storage/DAM system
Annotate
Automatic Tracing & Referencing
Annotate excels at tracing references and substantiating content. That task can now be achieved within seconds, without the hours of fact finding and researching. Start focusing on what really matters- delivering high quality content to your audience.
Instantly Trace Content to References
Eliminate manual searching by linking claims and reference to source documents with one click.
Speed Up Content Creation and MLR Submission
Reduce friction between creators and reviewers by embedding substantiation early in the workflow—no last-minute delays.
Accelerate MLR Cycles with High Quality Submissions
Ensure every submission is complete, compliant, and easier for reviewers to approve—cutting down on rounds of revision.
"Mindblown."
-Content Owner, Large Pharma
"Time Saver."
-Content Owner, Large Pharma
"...like magic"
-Copywriter Marketing Agency
- Export directly to PDF
- Mark up content and comment on annotations to speed review
- Reduce manual effort and mistakes
Screen
MLR Expertise at Your Fingertips
Screen serves as your personal brand guardian. Screen easily converts screenshots to text, spots and offers corrections for inconsistencies, and keeps your content in line so it can be approved more efficiently.
Slash Rework Costs by 85%
Catch issues early—before they hit MLR—reducing expensive rework cycles and saving your brand serious budget.
Save 80% of the Time on QA Checks
Instantly detect inconsistencies in claims, references, safety language, and editorial—no more manual comb-throughs or missed details.
Activate AI Across Your Entire Workflow
Easily turn on Screening Agent for every team—content creators, editors, reviewers, and approvers—so quality control becomes everyone’s superpower.
- Actionable feedback for error correction
- Collaborate across teams and departments
- Customize reviews to give you the answers you’re looking for
Integrations
Access your agents in tools you already love.
Generative technology is a great time saver for content creation, but in the life sciences space it’s important not to stray from what’s compliant and approvable. Reduce the risk of using generative design tools by utilizing Lithero plugins and API connections.
Figma®
Access Claims and Screen directly in your Figma projects, enabling you to create compliant content in real time.
 More Details Here
More Details HereAdobe® Suite
Complement Adobe generative design tools with Lithero to ensure content is high quality and accurate.
 More Details Here
More Details HereMicrosoft® Word
Open Claims and Screen directly in Microsoft Office, enabling you to create compliant content in real time.
 More Details Here
More Details HereFrom First Draft to Final Approval
Meet rising demand without lowering quality.
CHECK OUT OUR CASE STUDYUse Cases for Pharma:
Grow Brands. Reach Patients.
(Efficiently and Cost Effectively)
Lithero Agents boost commercial teams—delivering compliant, omnichannel content faster, smoother, and smarter than ever before.
Accelerate Speed-to-Market
Cut approval timelines from weeks to days, ensuring your brand stays competitive in fast moving therapeutic markets.
Accelerate Speed-to-Market
Cut approval timelines from weeks to days, ensuring your brand stays competitive in fast moving therapeutic markets.
Reduce Cost of Content Delivery
Scale assets without scaling headcount—lowering agency spend and operational overhead.
Reduce Cost of Content Delivery
Scale assets without scaling headcount—lowering agency spend and operational overhead.
Compliance Confidence
Integrate regulatory rules into the workflow, minimizing MLR cycles and protecting against costly delays.
Compliance Confidence
Integrate regulatory rules into the workflow, minimizing MLR cycles and protecting against costly delays.
Omnichannel Agility
Enable your commercial teams to engage HCPs and patients with consistent, on-brand content across digital, print, and field channels.
Omnichannel Agility
Enable your commercial teams to engage HCPs and patients with consistent, on-brand content across digital, print, and field channels.
Unlock Strategic Capacity
Free up your marketers and medical teams to focus on insights and strategy, not chasing revisions and approvals.
Unlock Strategic Capacity
Free up your marketers and medical teams to focus on insights and strategy, not chasing revisions and approvals.
Lithero Partnerships
Lithero provides cutting-edge technology solutions specifically designed for life sciences and pharmaceutical companies. Through strategic partnerships, we deliver exceptional customer experiences for clients across the globe.
As strategy specialists and trusted partners, you’re at the forefront of helping your Life Sciences clients identify and implement AI tools that give them competitive advantage and deliver significant ROI. LARA can play a crucial role in driving that value for your clients. Here's how you benefit from partnering with Lithero:
- Surprise and delight your clients with proven AI innovation
- Show measurable ROI for your clients fast
- Make a big impact with fewer resources as Lithero Agents don't require heavy implementation, your team can focus on strategy, transformation, and change readiness
- Enhance your own content service offerings powered by Lithero AI
Interested in partnering? Reach out: hello@lithero.com
Use Cases for Consultants:
Empower Clients. Differentiate Your Practice.
Win together with Lithero.
With Lithero Agents in your toolkit, you can deliver faster wins for pharma clients in cost management, content velocity, digital transformation, and AI adoption—while positioning your firm as the partner that drives measurable impact.
Accelerate Client Impact
Move from endless revisions to rapid approvals, so your creative work hits the market on time.
Accelerate Client Impact
Move from endless revisions to rapid approvals, so your creative work hits the market on time.
Reduce Compliance Headaches
Lithero Agents build in regulatory guardrails, cutting down friction with client MLR teams.
Reduce Compliance Headaches
Lithero Agents build in regulatory guardrails, cutting down friction with client MLR teams.
Scale Without Burnout
Transform your clients' operations by scaling efficiencies fast instead of enabling pilot fatigue.
Scale Without Burnout
Transform your clients' operations by scaling efficiencies fast instead of enabling pilot fatigue.
Differentiate Your Firm
Win new business by showing clients you bring AI-powered solutions, not just concepts and frameworks.
Differentiate Your Firm
Win new business by showing clients you bring AI-powered solutions, not just concepts and frameworks.
Strengthen Client Loyalty
Deliver measurable speed and efficiency gains that make you indispensable to pharma commerical leaders.
Strengthen Client Loyalty
Deliver measurable speed and efficiency gains that make you indispensable to pharma commerical leaders.
Interested in partnering? Reach out: hello@lithero.com
Use Cases for Agencies:
Deliver More. Stress Less. Win Bigger.
Supercharge your output.
With Lithero’s team of AI Agents, your agency can deliver faster, compliant, omnichannel content for pharma clients—helping you stand out, scale campaigns, and grow client relationships.
Accelerate Campaign Delivery
Move from endless revisions to rapid approvals, so your creative work hits the market on time.
Accelerate Campaign Delivery
Move from endless revisions to rapid approvals, so your creative work hits the market on time.
Reduce Compliance Headaches
Lithero Agents build in regulatory guardrails, cutting rework and approval friction for your teams.
Reduce Compliance Headaches
Lithero Agents build in regulatory guardrails, cutting rework and approval friction for your teams.
Scale Without Burnout
Take on more client projects without overloading your staff or ballooning costs.
Scale Without Burnout
Take on more client projects without overloading your staff or ballooning costs.
Differentiate Your Agency
Win new business by showing clients you bring AI-powered solutions, not just creative.
Differentiate Your Agency
Win new business by showing clients you bring AI-powered solutions, not just creative.
Strengthen Client Loyalty
Deliver measurable speed and efficiency gains that make you indispensable to pharma brands.
Strengthen Client Loyalty
Deliver measurable speed and efficiency gains that make you indispensable to pharma brands.
Interested in partnering? Reach out: hello@lithero.com
What makes Lithero Different?
Agents Built for the Entire Content Supply Chain -
Not Just One or Two StepsOur agents capture brand claims, check compliance, aid modular content creation and offer relevance insights.
Get Up and Running at Lightspeed
Onboarding is straightforward and rapid- we’re talking days.
Automated Brand-Specific Encyclopedia
Our Claims agent identifies up to 95% of brand-specific compliance and editorial errors, while creating an auto-maintained centralized source of truth for claims and references.
Integrate Directly to Tools You Love
Get tailored insights and instant access to brand claims and references right in your content creation tools as you work.
Ready to streamline every step of your content process? See how Lithero delivers efficiency from concept to compliance. Start your free trial today.
START HERE